Zydus Cadila's ZyCoV-D - the needle-free COVID-19 vaccine for adults and kids - all you need to know
India on Friday (August 20, 2021) granted emergency use approval for Zydus Cadila's COVID-19 vaccine 'ZyCoV-D', which is the world's first DNA shot against the coronavirus in adults and children aged 12 years and above.
)
Zydus Cadila had applied for the emergency use authorization of ZyCoV-D in July. It was based on an efficacy rate of 66.6% in a late-stage trial of over 28,000 volunteers nationwide. This has been reportedly the largest COVID-19 vaccine trial so far in India.
(Photo: Zydus Cadila)
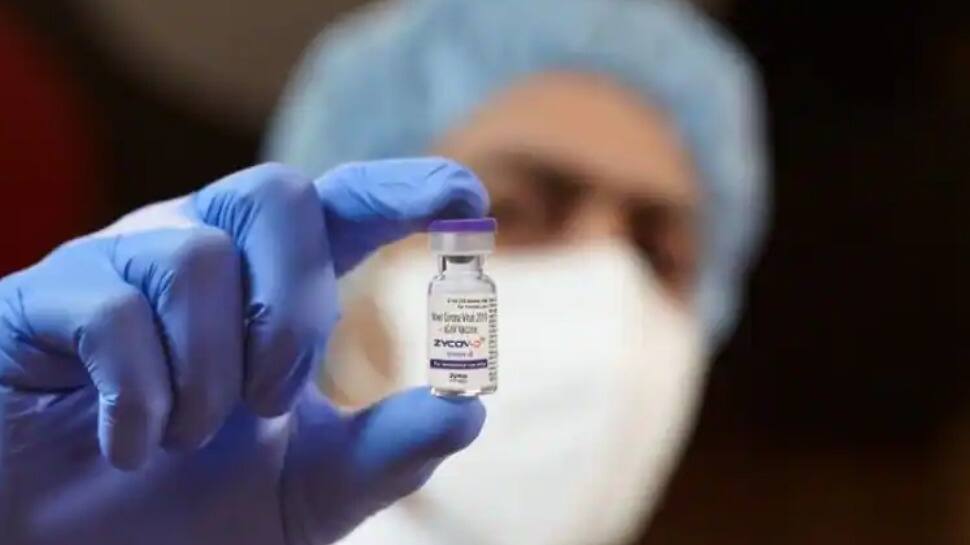
ZyCoV-D is a three dose coronavirus vaccine that will be administered first on day zero, then on day 28th and on the 56th day.
(Photo: Zydus Cadila)

ZyCoV-D is a needle-free COVID-19 vaccine which is administered using The PharmaJet, a needle-free applicator, which ensures painless intradermal vaccine delivery. This will majorly help in administering the vaccine to kids.
(Photo: IANS)

Being a Plasmid DNA vaccine, ZyCoV-D doesn't have any problem associated with vector-based immunity. The Plasmid DNA platform also allows generating new constructs quickly to deal with mutations in the virus.
(Representational Photo: Pixabay)

ZyCoV-D is stored at 2-8 degrees C and has shown good stability at temperatures of 25 degrees C for at least three months.
(Photo: Zydus Cadila)

As per Zydus Cadila, the firm is planning to manufacture 10-12 crore vaccine doses of ZyCoV-D annually.
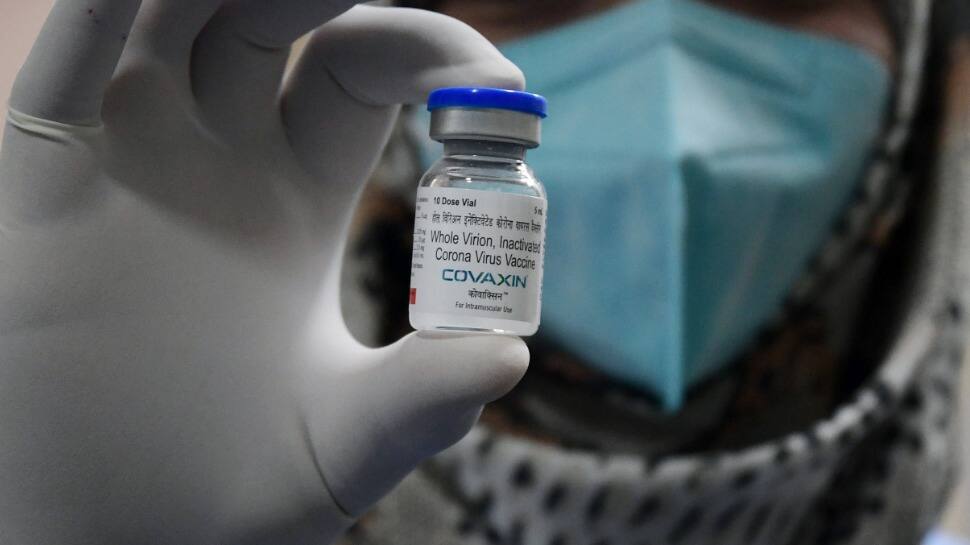
Zydus Cadila's coronavirus vaccine is the second home-grown shot to get emergency authorization in India after Bharat Biotech's Covaxin. It is developed in partnership with the Department of Biotechnology.
ZyCoV-D has also become the sixth COVID-19 vaccine to get approval in India.
(Representational Photo: ANI)

